Anthony Karabanow, MD
Cutaneous anthrax is endemic in Haiti. Colloquially known as “malcharbon” (or “sick charcoal,”) this disease has been responsible for multiple regional outbreaks in Haiti. An outbreak of cutaneous and systemic disease involving > 100 cases and >12 deaths occurred in La Brillere in 1996. In 1988, the Haitian Ministry of Health recorded 164 cases of anthrax in the Commune of Jeremie1.
Anthrax is caused by the spore forming bacteria Bacillus anthracis. As spores, this organism can survive for extended periods in the environment. Indeed, anthrax spores are often part of normal soil flora. Under certain circumstances (e.g. after abundant rainfall or drought,) the bacteria can undergo a burst of replication. Unfortunately, cycles of flooding followed by drought (“anthrax weather”) have become more common in Haiti as a result of deforestation. Flooding and subsequent run-off concentrates anthrax spores in low-lying areas; drought evaporates pooled water and further concentrates the spores2.
Domestic animals (cattle, sheep, goats, pigs, horses) may ingest the spores while grazing. Anthrax in animals results in a syndrome of fever, breathing difficulty, convulsions and bleeding from multiple orifices. Death occurs in 2-3 days in untreated animals. Humans acquire this disease by coming into contact with infected animals. Anthrax has long been recognized as an occupational hazard for those who handle animal hides, veterinarians and agricultural workers.
Human anthrax usually involves the skin (most often of the neck, face and upper extremities.) Spores typically enter the skin through minor cuts or abrasions. The spores then germinate, multiply and release toxins. Clinical disease is actually the result of toxins released by the organism. Most cutaneous lesions remain localized and resolve spontaneously in a matter of weeks. However, in 20 percent cases, cutaneous anthrax can spread into the blood stream and result in sepsis, meningitis and death. This justifies treating all cases of cutaneous anthrax with antibiotics. With prompt antibiotic therapy, mortality from cutaneous anthrax is virtually zero.
Anthrax can also be acquired through inhalation and the GI tract. These forms are far less common than cutaneous anthrax and usually misdiagnosed. Inhalational anthrax (“woolsorters disease”) may occur in those who inhale spores while working with the hides of infected animals. The initial symptoms are those of a viral upper respiratory infection (i.e. fever, non-productive cough, myalgias and malaise.) A chest x-ray performed early in the disease shows a widened mediastinum from hemorrhagic mediastinitis. Patients ultimately succumb to respiratory failure and shock despite antibiotics.
Gastrointestinal anthrax results from the consumption of meat from infected animals. This results in the formation of ulcers in the digestive tract similar to those found on the skin. Symptoms include fever, abdominal pain and bloody stools/vomit. Ascites often develops. Ascitic fluid analysis often reveals the presence of anthrax. Mortality (due to intestinal perforation or sepsis) ranges from 4 to 60% in various studies.
The incubation period of cutaneous anthrax is typically 5-7 days. The first stage of the disease is a painless (but often pruritic) papule. This enlarges and develops a central vesicle. Ultimately, a painless ulcer with a central black, depressed eschar forms. There is often surrounding tissue edema and regional lymphadenopathy. Fever, malaise and headache may also be present. Smego and Gebrian described and photographed several cases of cutaneous anthrax at a rural clinic near Jeremie1:
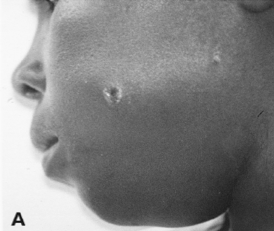
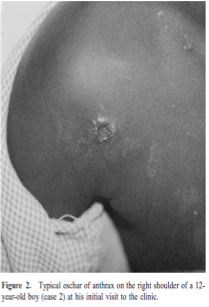 In Haiti, where microbiology resources are scarce, the diagnosis of cutaneous anthrax will be based on clinical suspicion and gram staining. Gram staining may be performed on an unroofed vesicle (dry swab), base of ulcer (moist swab) or at the edges/underneath an eschar (moist swab). Anthrax stains as a gram positive rod.
In Haiti, where microbiology resources are scarce, the diagnosis of cutaneous anthrax will be based on clinical suspicion and gram staining. Gram staining may be performed on an unroofed vesicle (dry swab), base of ulcer (moist swab) or at the edges/underneath an eschar (moist swab). Anthrax stains as a gram positive rod.
The treatment of cutaneous anthrax is as follows:
• Oral ciprofloxacin: 500 mg (15 mg/kg) PO BID in adults (children) OR
• Oral doxycycline: 100 mg (2.2 mg/kg) PO BID in adults (children)
Penicillin based regimens (including amoxicillin) have traditionally been used but resistance is mounting. The duration of therapy is typically 7 days. Excision of the lesions is contraindicated as this may precipitate systemic dissemination.
It is important to understand that although antibiotics will rapidly kill off the organism, progression of the lesion to the eschar stage will continue. This is because such development is the result of toxins already elaborated by the organism.
Peck and Fitzgerald examined the incidence of cutaneous anthrax at Albert Schweitzer Hospital in the Artibonite Valley between 1992 and 20022. They reported an annual incidence that varied between 4 and 72 cases per million. They identified the following risk factors for acquiring this disease:
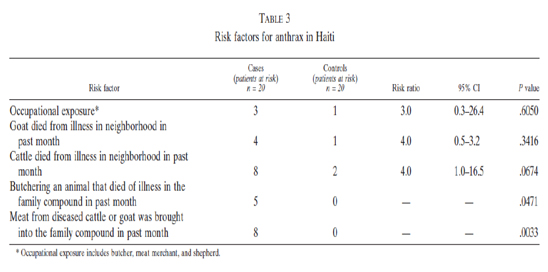
They also described Haitian butchery practices that might explain the high incidence of the disease on the face and among children:
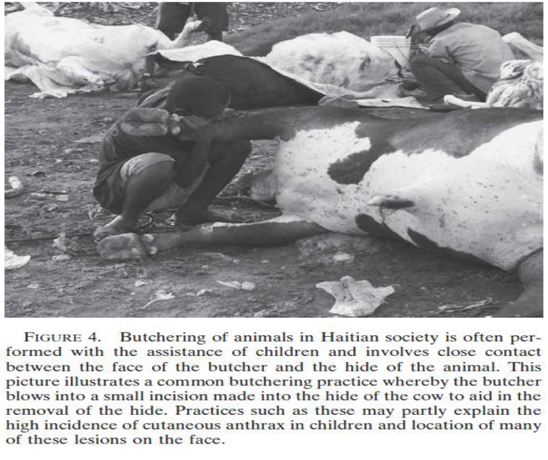
In their article, Smego and Gabrian attributed peri-oral anthrax to the practice of children suckling an animal’s teat while playing outdoors.
Peck and Fittzgerald also noted a number of deaths secondary to rapidly fatal airway obstruction. This appeared to be caused by external compression of the airway from severe facial edema. Thus cutaneous anthrax involving the head or neck should prompt rapid intervention and close airway monitoring on the part of the health care provider. Steroids may be added to antibiotic therapy in such severe cases.
Haitian law mandates the vaccination of domestic livestock and the burning of any animal suspected of having anthrax. Unfortunately, poverty and lack of enforcement have thwarted these public health measures in Haiti.
References:
1. Smego RA and Gebrian B., “Cutaneous manifestations of anthrax in rural Haiti.” Clin Infect Dis., 1998, 26(1): 97-102.
2. Peck RN and Fitzgerald DW. “Cutaneous anthrax in the Artibonite Valley of Haiti: 1992-2002,” Am J Trop Med Hyg., 2007, Nov; 77(5):806-11.
3. Dixon CT et al., “Anthrax,” NEJM, 1999, 341 (11): 815-826.