Anthony Karabanow, MD
This article is designed to introduce the health care practitioner to the diagnosis and treatment of HIV in Haiti based on available resources at Hôpital Sacré Coeur (HSC) and according to Haitian national HIV standards. When newer WHO standards exist, these have been included preferentially to older Haitian national standards. The treatment of patients co infected with HIV and TB will also be covered.
According to the USAID website:
“With 2.2 percent of adults estimated to be HIV positive, Haiti has a generalized HIV/AIDS epidemic. It has the largest epidemic in the Caribbean, where about three-quarters of HIV-positive people live in Haiti or the Dominican Republic. First reported in 1979, HIV infections in Haiti increased until the early 1990s. Prevalence then began to decrease, particularly in urban areas. HIV prevalence among pregnant women attending antenatal clinics declined notably from 5.9 percent in 1996 to 3.1 percent in 2004. The decline, however, appears to have stabilized at 2.2 percent prevalence in recent years.The latest sentinel antenatal survey found a seroprevalence rate among pregnant women of 2.7 percent. While positive behavior changes may be in part responsible for the overall decline, significant levels of high-risk behavior have persisted, particularly in rural areas and among young people. Overall, the negative health, economic, and social impacts of HIV/AIDS continue to be disproportionately high due to a weak health care system, extreme poverty, and AIDS-related stigma and discrimination.”
DIAGNOSIS:
At Hôpital Sacré Coeur, HIV is diagnosed via a rapid HIV test. In this test, HIV antigens are affixed to a test strip. The strip is exposed to a sample of blood obtained via finger stick. If HIV antibodies are present in the blood sample, they bind to the affixed antigen. This results in an indicator change. This test has sensitivity and specificity in the high 90th percentile. A western blot for confirmation is not available. The HSC lab can determine the CD4 count but not the viral load.
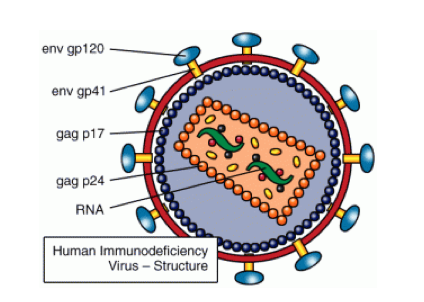 ANTI RETROVIRAL TREATMENT (ART):
ANTI RETROVIRAL TREATMENT (ART):
According to the WHO, a CD4 count < 350 is the threshold to initiate ART. For those in whom ART is deferred, follow up CD4 counts should be obtained every 6 months.
Current antiretroviral therapy consists of a “base” and a “backbone.” The base consists of either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI.) The backbone consists of 2 nucleoside reverse transcriptase inhibitors (NRTI.)
| The HIV medications available at HSC are as follows: |
The WHO is trying to phase out d4T use due its toxicity (lipodystrophy, lactic acidosis and neuropathy.) Thus, this agent will not be discussed further.
The dosing of HIV medication is as follows:
NVP (200 mg): 1 tab PO QD x 2 weeks, then 1 tab PO BID
EFV (200 mg): 3 tabs PO QHS (on empty stomach)
LPV/r (133mg/33mg): 3 tabs PO BID
AZT + 3TC (300 mg + 150 mg): 1 tab PO BID
TDF (300 mg): 1 PO QD
FTC (200 mg): 1 PO QD
3TC (150 mg): 1 PO BID
First line therapy for previously untreated adults/adolescents consists of an NNRTI and 2 NRTIs:
EFV/NVP
+
AZT + 3TC OR TDF + 3TC/FTC
NVP and EFV have comparable efficacy. NVP has a higher incidence of rash, Stevens-Johnson syndrome and hepatotoxicity. NVP is the preferred agent for women who are or may become pregnant. There are reports of an increased risk of liver toxicity in women when the CD4 count is 250 – 350. The WHO HIV guideline committee found that there was limited cause for concern but recommended close monitoring of liver enzymes during the first 12 weeks of therapy. All patients on NVP should have liver enzymes assessed prior to treatment and serially thereafter. The side effect profile of EFV includes CNS toxicity (which typically resolves in 2-4 weeks,) rash and possible teratogenicity if taken during the first trimester of pregnancy. Thus, this agent should be avoided in those with a history of severe psychiatric disease and during the first trimester of pregnancy. AZT can cause anemia and neutropenia. As a result, the CBC needs to be assessed prior to treatment and serially thereafter. It should not be given in those with an Hg < 7. Anemia is common co morbidity in Haiti due to malaria, parasites, malnutrition and repeated pregnancies. A low CD4 count is also a risk factor for anemia. TDF can result in renal dysfunction. As a result, the serum creatinine needs to be assessed prior to treatment and serially thereafter.
FOLLOW UP:
Upon initiation of ART, patients are seen every 1-2 weeks for the first 3 months. Each visit is an opportunity to evaluate patient comprehension, compliance and to evaluate for adverse drug reactions. Thereafter, patients are initially seen monthly. The CD4 count is followed every 6 months.
HIV Treatment Failure:
At this time, Hôpital Sacré Coeur is unable to perform viral load studies. Thus, treatment failure is defined in immunologic terms:
• Fall of CD4 count to baseline
• 50% fall of CD4 count from peak treatment value
• Persistent CD4 count below 100
Treatment failure usually mandates a change in treatment regimen to a PPI and different NRTIs:
LPV/r
+
AZT + 3TC OR TDF + 3TC/FTC
LPV/r is associated with hepatotoxicity, hyperglycemia and hyperlipidemia. Thus, there is a need for hepatic enzyme, blood sugar and lipid follow-up.
Cotrimoxazole Prophylaxis:
TMP-SMX prophylaxis is recommended for all patients with CD4 count < 350 given the concurrent risks of malaria and bacterial infection. It is also provided to all HIV-TB co infected patients.
HIV and TB Co infection:
This is a common occurrence and needs to be anticipated. All patients diagnosed with HIV need to be screened for TB (and vice versa). All HIV patients with TB should be started on ART irrespective of CD4 counts.
Asymptomatic patients are screened via a PPD. Patients with possible active TB are evaluated via serial sputum AFBs and a CXR. Latent TB patients with HIV are treated with isoniazid preventative therapy. This consists of INH + B6 for 6 months. Active TB patients with HIV are treated with same anti-TB regimens as for those without HIV. TB therapy should begin prior to ART.
Patients with TB should be started on ART between 2 and 8 weeks after beginning TB therapy. This is because the case-fatality amongst HIV-TB patients occurs mainly in the first 2 months of TB treatment. Unfortunately, this results in an increased pill burden, the potential for drug interactions and IRIS (immune reconstitution inflammatory syndrome.) IRIS refers to a paradoxical worsening of a pre-existing infection due to functional restoration of CD4 cells. This syndrome is common in patients with TB who are started on ART. The syndrome can present as fever and worsening pulmonary infiltrates.
Due to interactions with rifampin, the preferred ARV for those co infected is an EFV based regimen. If this is not tolerated (or contraindicated as in pregnancy,) NVP should be used (without a lead-in dose.) For those who need to use LPV/r, rifampin must be changed to rifabutin at a dose of 150 mg three times per week. The LPV/r regimen should contain “super boosted” RTV (i.e. 800/200 BID.)
HIV and pregnancy:
All pregnant women need to be screened for HIV due to the risk to them and for maternal-fetal transmission. ART is recommended in HIV positive pregnant women with CD4 < 350. The preferred regimens are the same as for non-pregnant women. However EFV should be avoided in the first trimester. In this scenario, infants are given daily NVP or twice daily AZT from birth until 4-6 weeks of age (irrespective of feeding mode.)
For women not requiring ART for their own health, there are 2 possible regimens:
OPTION A:
1) Ante partum AZT from as early as 14 weeks gestation
2) Single dose NVP at onset of labor
3) AZT + 3TC during labor and delivery
4) AZT + 3TC for 7 days after delivery
Note that NVP and AZT+3TC can be omitted if the mother receives > 4 weeks AZT ante partum. In this case, continue AZT during labor and stop at delivery.
Breast feeding infants receive daily NVP from birth for a minimum of 4 to 6 weeks and until 1 week after all exposure to breast milk. Non-breast feeding infants receive daily NVP or single dose NVP + twice daily AZT from birth until 4-6 weeks.
OPTION B:
Triple ARV* prophylaxis starting as early as 14 weeks gestation and continued until delivery. If breastfeeding, continue until 1 week after all infant exposure to breast milk.
Irrespective of feeding mode, infants receive daily NVP or twice daily AZT from birth until 4-6 weeks.
*Triple ARV = AZT + 3TC + LPV/r OR AZT + 3TC + EFV OR TDF + FTC/3TC + EFV
Post –exposure prophylaxis:
The risk of HIV infection after exposure to HIV infected body fluids is generally low (0.33 %) after needle stick. It is even lower after mucosal exposure (0.09 %.) However, this data was obtained in resource-rich countries with a low prevalence of HIV and may not apply to Haiti. The risk of HIV acquisition is related to the nature of the exposure:
Low risk: Superficial injury from a solid need (ex suture needle,) mucocutaneous exposure.
High risk: From a hollow bore needle with visible blood, or from a needle previously in an artery or vein.
In case of needle sticks, the following protocol should be followed:
1) Wash out the wound with soap and water. If available, also use an alcohol based hand hygiene solution or chlorhexidine as these are virucidal to HIV.
2) Ascertain HIV status of source patient by a rapid HIV test.
3) Obtain a baseline HIV test at 0, 3 and 6 months for the exposed party.
4) If the rapid HIV test in the source patient is positive, initiate prophylaxis with AZT +3TC for 4 weeks. This regimen should be begun ASAP and certainly no later than 72 hours after exposure. If there is reason to suspect resistant HIV in the source patient, add LPV/r.
References:
1. Manuel de Normes de Prise en Charge Clinique et Thérapeutique des Adultes et Adolescents Vivant avec le VIH, (HAITI 2008.)
2. “Antiretroviral therapy for HIV infection in adults and adolescscents,” (WHO 2010.)
3. “Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants.” (WHO 2010.)
4. USAID Website Haiti.
5. “Guidelines for intensified tuberculosis case finding and isoniazid preventative therapy for people living with HIV in resource-constrained settings,” (WHO 2011.)